Products

Who We Serve
October 4, 2018
The 2019 IPPS Final Rule was issued by the Centers for Medicare and Medicaid Services (CMS) in August 2018. According to CMS the goal of this final rule was to “further advance the agency’s priority of creating a patient-centered healthcare system by achieving greater price transparency, interoperability, and significant burden reduction so that hospitals can operate with better flexibility and patients have what they need to be active healthcare consumers.”
At 600+ pages, most of us don’t have time to read and decipher it in its entirety. Don’t worry, we’ve got you covered! Our experts (a special thank you to Michelle K!) have broken down the major changes and what you need to know about the 2019 IPPS Final Rule…
Meaningful Use had a name change, and the new name is Promoting Interoperability (or PI). So, while the program is still active, the name change further emphasizes the heavy focus on interoperability between disparate systems. The new name applies to both Medicare and Medicaid programs.
While there were several changes to measures, we’ve only highlighted some of the major ones here:
NEW Measures
There are 2 new ePrescribing measures related to opioids to help combat the rising opioid epidemic:
By extending the optional reporting status for these measures, CMS is giving providers extra time to put an opioid treatment plan in place.
REMOVED Measures
Overall, CMS reduced the total number of measures acute care hospitals are required to report across the 4 quality and value-based purchasing programs (IQR, Value-Based Purchasing, Hospital-Acquired Conditions Reduction, and Readmissions Reduction Programs). A total of 18 measures were removed that did not emphasize interoperability and the electronic exchange of health information, and another 25 measures were de-duplicated. In removing these measures, CMS focused on those that were duplicative, overly costly to maintain and report, or where the majority of providers were already performing highly.
A full list of the removed (43) measures and rationale for removing them is found here.
A new performance-based scoring methodology was introduced whereby CMS essentially took Stage 3 methodology and removed, added, modified, or maintained a smaller set of various objectives and measures to come up with this new score. There are 100 points possible, and as long as you score a minimum of 50/100 points, or 50%, you ‘pass’ and are classified as a meaningful user of certified EHR technology (CEHRT). The purpose of this change was to provide eligible hospitals/CAHs with an easier and more flexible structure so they can focus more on patients.
As part of the simplified structure, starting in 2019, CMS limited the number of objectives to only 4. This means Eligible Hospitals and CAHs will be required to report measures from 4 objectives with scoring at the individual measure level:
Example of CMS New Performance-Based Scoring for CY 2019:
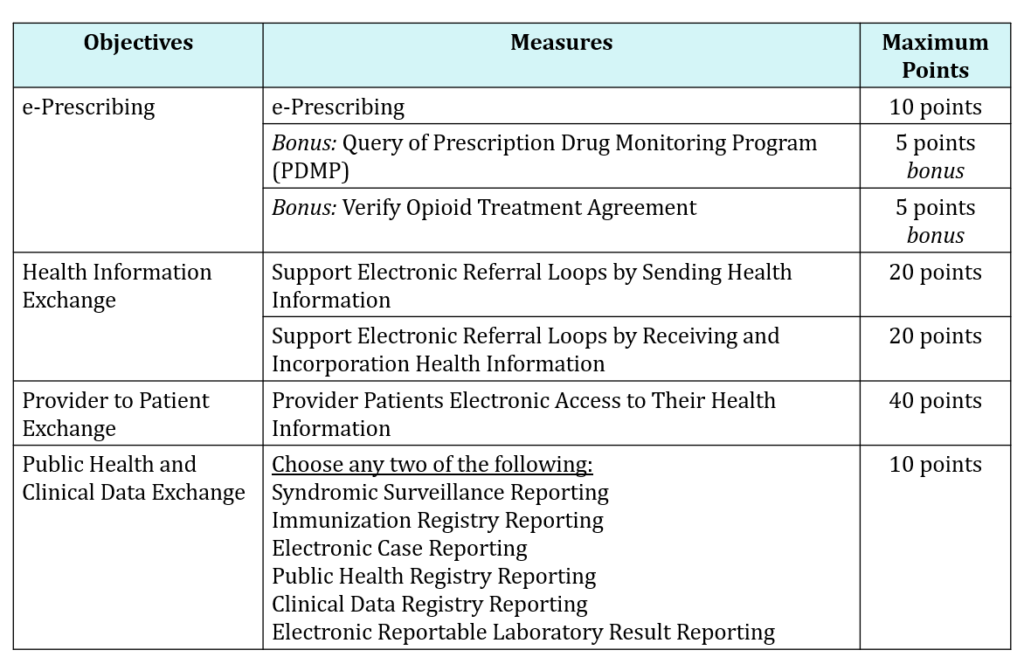
It’s important to note a few things: A Security Risk Analysis is still required but is not included as part of the scoring methodology. In addition to submitting a Security Risk Analysis, you must also submit complete numerator and denominator metrics or yes/no data for all required measures, and report on all of the required measures across all of the objectives in order to earn any score at all. Failure to report any required measure, or reporting a “no” response on a yes/no response measure (unless an exclusion applies) will result in a zero. You also must submit a numerator of at least one patient for the measures that require a numerator and denominator.
2020 Reporting Period Changes
The only changes for 2020 are for the e-Prescribing objectives:
One of the more exciting changes is allowing new and returning participants to utilize any continuous 90 days for a reporting period for the 2019 and 2020 calendar years. This was done so that providers can become familiar with new measures and the new scoring methodology.
Eligible Hospitals and CAHs that submit eCQMs electronically can continue to select a minimum of 4 CQMs from any calendar year quarter to report on (no change from the 2018 reporting period). However, for 2020, 8 of the 16 available CQMs will be removed because CMS feels that the costs associated with these measures outweigh the benefit of their continued use in the program. As an example, ED-1 was removed because CMS feels that ED-2 is more effective at driving quality improvement. You will still be able to use ED-1 and the other 7 measures listed below through 2019, but beginning with 90-day reporting period in 2020 (whatever you choose that to be), you will need to choose other measures to replace some of the 4 you may have already been reporting on.
Removed eCQMs as of 2020 Reporting Period:
CMS also stressed the importance of using a 2015 Edition of CEHRT, starting with an EHR reporting period in CY 2019. For your convenience, a full list of certified health IT products can be found here: https://chpl.healthit.gov/#/search. Wellsoft EDIS is already 2015 Edition certified, and continues to work on adding more criteria to our certified status!
Stay tuned for our next blog post on the importance of Usability as it relates to 2015 Edition Certification, and see how ED EHR vendors stack up.
Still have questions regarding the Final Rule? Contact us to let us know and we’ll be in touch shortly.